Showing posts with label Spheroidal Shape. Show all posts
Showing posts with label Spheroidal Shape. Show all posts
Thursday, January 3, 2013
Tetrahedral C28 and related structures
There are only three tetrahedral fullerenes with number of carbon atoms less than that of buckyball. They are C28, C40, and C44, respectively. The spiral code for the smallest tetrahedral fullerene, C28, is [1 2 3 5 7 9 10 11 12 13 14 15]. Following this code, we can easily make its bead model using the standard figure-eight stitch. We can see that, in this molecule, there are 12 pentagons, 3 in a group located at a vertex, and 4 hexagons located on the four faces of the tetrahedron. If we replace these pentagons by heptagons, we get a tetrapod-like structure, in which tri-pentagon vertices become tri-heptagon necks as shown in the following figure.
Using these tetrapods as building blocks, we can get the following diamond-like structure. In fact, this is exactly the structure Mr. Horibe put in the postcard. OK, if we start from other tetrahedral fullerenes such as C40 and C44, we can find out a lot more diamond-like structures.
Monday, July 9, 2012
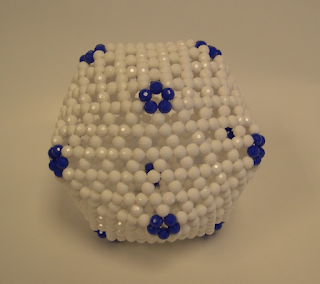
Truncated octahedron
C60 and extended
C168 are unique because neighbored nonhexagons in them are separated by exactly one carbon-carbon bond. Is there any other graphitic structure with the similar property? The answer is yes. Chern and I have written an article on the carbon nanotori and nanohelices with this property a few years ago.
Chuang, C; Jin, B.-Y.* “Hypothetical Toroidal, Cylindrical, Helical Analogs of C60.” J. Mol. Graph. Model. 2009, 28, 220-225.
Of course, it is easy to see that there are another four Archimedean solids with this property if we allow nonhexagons to be squares or triangles. They are truncated octahedron (see the following photo), truncated cube, truncated tetrahedron, and truncated dodecahedron. Note that C60 is the truncated icosahedron. So all five truncated Platonic solids belong to this class.
Chuang, C; Jin, B.-Y.* “Hypothetical Toroidal, Cylindrical, Helical Analogs of C60.” J. Mol. Graph. Model. 2009, 28, 220-225.
Of course, it is easy to see that there are another four Archimedean solids with this property if we allow nonhexagons to be squares or triangles. They are truncated octahedron (see the following photo), truncated cube, truncated tetrahedron, and truncated dodecahedron. Note that C60 is the truncated icosahedron. So all five truncated Platonic solids belong to this class.
Tuesday, July 3, 2012
Carbon cube
Using the same combination of an octagon together with four pentagons for a face as described in the carbon cuboctahedron, one can make the following bead model of the carbon cube. Each corner of a cube is mimicked by three fused pentagons. In principle, we can use the same strategy to create a whole series of fullerenes with the shape of a truncated cube.
Monday, July 2, 2012
Carbon cuboctahedron
Most of cage-like fullerenes belong to either icosahedral or tetrahedral groups. But if we remove the restriction of using only pentagons and hexagons, we can create cage-like fullerenes with octahedral symmetry (or cubical shape) too. Of course, all P-type TPMSs, which are extended systems, posted before have the same symmetry. But I have never made cage-like fullerenes with cubical shape before. I found a few examples of cage-like fullerenes with cubical shape in an interesting book with the title, Periodic Nanostructures, by M. V. Diudea and C. L. Nagy recently. In this structure, each face contains an octahedron surrounded by four pentagons, which give a topological charge of 2. There are six faces in a cube, so the total topological charge is 12 as required by the Euler theorem. It is also easy to see that the eight vertices of this molecule are covered by flat coronenes. Therefore, the molecule looks like a cuboctahedron (立方八面體).
It is not hard to see that one can grow eight carbon nanotubes along eight vertices of the cube. The result will be a Schoen's I-WP surface I described before. If one inserts six tubes along the six faces, one get a single unit cell of the P-surface. Or one can also terminate the CNTs to get a dendritic fullerene with a cubic-shape core.
It is not hard to see that one can grow eight carbon nanotubes along eight vertices of the cube. The result will be a Schoen's I-WP surface I described before. If one inserts six tubes along the six faces, one get a single unit cell of the P-surface. Or one can also terminate the CNTs to get a dendritic fullerene with a cubic-shape core.
Friday, June 8, 2012
Construction procedure of C60 (Japanese translation)
Prof. Sonoda made a nice Japanese translation of the detailed construction described in the supporting information of the article, Molecular Modeling of Fullerenes with Beads, J. Chem. Edu 2012 , 89 (3), 414–416.", by Prof. Cuccia and me.



Also, here is the original English version of the first page, the next two pages are the same.




Also, here is the original English version of the first page, the next two pages are the same.

Japanese translation of 珠璣科學─串珠碳六十
Prof. Inoue (井上吉教) of Hikone prefecture university and one of his Chinese students kindly translated my article "珠璣科學─串珠碳六十" (The Science of Beading - Beaded C60) with Dr. Tsoo for the March issue of the "Science Monthly (科學月刊)" magazine.

(New post, scanned images 6/12/2012)





Prof. Inoue also made a few fullerenes as shown in the following pictures. I didn`t check them very carefully. Possibly, one of them is the icosahedral C80 and another is the cylindrical shape C84. There seems to be a fullerene belonging to D3h point group.



(New post, scanned images 6/12/2012)





Prof. Inoue also made a few fullerenes as shown in the following pictures. I didn`t check them very carefully. Possibly, one of them is the icosahedral C80 and another is the cylindrical shape C84. There seems to be a fullerene belonging to D3h point group.


Friday, June 1, 2012
C76
There are two isomers of C76 that satisfy the isolated pentagon rule (IPR). Here is the bead model for C76 with Td symmetry just constructed by Yuan-Chia Fan.
The spiral codes for these two isomers of C76 are
C76:1 [1 7 9 11 13 18 26 31 33 35 37 39] D2
C76:2 [1 7 9 12 14 21 26 28 30 33 35 38] Td
C76:1 [1 7 9 11 13 18 26 31 33 35 37 39] D2
C76:2 [1 7 9 12 14 21 26 28 30 33 35 38] Td
Sunday, April 15, 2012
Three Kekule structures of C60
D. Vukicevic and M. Randic have figured out all possible distinct resonance (or Kekule)
structures a few years ago. According them,
buckminsterfullerene has 12500 Kekule structures grouped in 158 isomorphic classes. They also give
a complete list of all these 158 non-isomorphic Kekuke structures in a recent paper
entitled "Detailed Atlas of Kekulé Structures of the Buckminsterfullerene", in the book,
"The Mathematics and Topology of Fullerenes".
This is very convenient if we want to make any particular resonance form of C60. We can simply look at the Schlegel diagrams given in this paper, and pay attention to the single and double bond pattern as we bead. Here are three bead models for Kekule structures No. 134, 135 and 136 as shown in their paper.
This is very convenient if we want to make any particular resonance form of C60. We can simply look at the Schlegel diagrams given in this paper, and pay attention to the single and double bond pattern as we bead. Here are three bead models for Kekule structures No. 134, 135 and 136 as shown in their paper.
Monday, April 2, 2012
beaded vase
Bang-Rui's mother knew my hobby of making beaded molecules. So she gave me a beautiful beaded vase she made. It is basically a pillar-shaped C84 with the top surface removed.
Here is a bead model of pillar-shape
C84 I made before.
The other two smaller fullerenes in the same homologous series are C60 and C72.
Monday, March 5, 2012
Pillar-shaped C60 and related C72
There are 1812 isomers of C60. Only the one with the shape of a soccer ball is stable enough and can be synthesized routinely. However, chemists in the Xiamen university have successfully created a few chlorinated isomers of C60 three years ago. I will try to make bead models of them when I find time. Here I would like to show the bead model of another elusive isomer of C60 with pillar shape (point group, D6h) which might be found someday.
Of course, there is a whole series of pillar-shape fullerenes by simply inserting short rings of carbon nanotubes. Here is the bead model of C72, which is the second one in this series.
Tuesday, February 28, 2012
C84 - a tetrahedral fullerene
I posted a few bead models of C84 before, but I had never given the detailed beading procedure for this molecule. C84 is the smallest achiral fullerene with tetrahedral shape that satisfies the independent pentagon rule (i.e. no two pentagons are connected).
Here is another bead model of C84 consisting of 8mm beads I made yesterday.
The easiest way to make it is to follow the beading path as shown in the following Schlegel diagram. Note that this path does not correspond to the path give by a spiral code.
In principle, one can puncture holes on this molecule and use the resulting structures as building blocks to create more complicated structures or super fullerenes (fused C84 in this case). I will show how this can be done later.
Here is another bead model of C84 consisting of 8mm beads I made yesterday.
The easiest way to make it is to follow the beading path as shown in the following Schlegel diagram. Note that this path does not correspond to the path give by a spiral code.
In principle, one can puncture holes on this molecule and use the resulting structures as building blocks to create more complicated structures or super fullerenes (fused C84 in this case). I will show how this can be done later.
Wednesday, October 12, 2011
C70 beading procedure
C70. Point group D5h.
Shape: like a Rugby ball.
60 8mm red beads and 45 8mm white beads are used.
Ring spiral: [1 7 9 11 13 15 27 29 31 33 35 37]



The red and green parts of spiral are exactly the same as the spiral of C60. The orange part of the spiral is a ring of 10 hexagons inserted in between two hemispheres of C60! Of course, instead of inserting one ring of 10 hexagons, one can do it repeatedly to get different length of endcapped carbon nanotubes! So C70 is the shortest endcapped carbon nanotube.
Note also that the blue circles in this graph are not beads! Beads represent edges or chemical bonds of fullerenes.
I made a few bead models for Prof. Gillespie, who proposed the the famous VSEPR method, last week. This C70 model is one of them.
Shape: like a Rugby ball.
60 8mm red beads and 45 8mm white beads are used.
Ring spiral: [1 7 9 11 13 15 27 29 31 33 35 37]



The red and green parts of spiral are exactly the same as the spiral of C60. The orange part of the spiral is a ring of 10 hexagons inserted in between two hemispheres of C60! Of course, instead of inserting one ring of 10 hexagons, one can do it repeatedly to get different length of endcapped carbon nanotubes! So C70 is the shortest endcapped carbon nanotube.
Note also that the blue circles in this graph are not beads! Beads represent edges or chemical bonds of fullerenes.
I made a few bead models for Prof. Gillespie, who proposed the the famous VSEPR method, last week. This C70 model is one of them.
Friday, October 7, 2011
C60 beading procedure
I took a few photos for making bead model of C60. This will be helpful for learning the so-called figure-eight stitch (or right-angle weave) and the beading rule of C60 I mentioned before.
South hemisphere:

North hemisphere:


* General instruction:
1. There are 32 polygons consisting of 12 pentagons and 20 hexagons in a C60.
2. Every pentagon is separated from neighbored pentagons by eactly one CC bond.
3. If we choose one color of beads for pentagons and the other for the rest, one would find hexagons made of two colors alternatively.
4. It is better to view C60 as a sphere consisting of six layers of polygonal strips. For the south semisphere, they are basically a pentagon for the south pole, five hexagons next, 10 polygons consisting of 5 pentagons and 5 hexagons. Reverse the beading sequence, one gets the north semisphere. (This is what people called the spiral code.)
5. One has to check how many beads of group one wants to create in the next step. Some beads are already done, so one has to thread the fishing cord through these beads first, then add the remaining beads through the other end of fishing cord, and finally, form the n-bead group by threading the fishing cord through the last bead just added along the opposite direction.
Of course, one should always check the sequence of colors of beads one is going to bead and make sure that they satisfy the color coding mentioned in 3.
For beginners, this is usually the hardest part and mistakes occur easily. Most often, wrong number of beads are added or some beads are not threading through first. But, if one can pay attention to the number of beads in the next group one is going to make, it should be trivial to figure out how many beads are already there and how many more one should add.
If two colors of beads are used, one can simply pay attention to color. Beading process for a C60 becomes trivial.
The beading procedure can be summarized by the spiral code on the following Schlegel diagram of C60. For C60, it is [1 7 9 11 13 15 18 20 22 24 26 32], which is essentially the positions of pentagons along the spiral path starting from south pole to the north pole. It is not hard to find this code is the only information we need to create a bead model of C60. Similarly, one can create any other cage-like fullerene by its spiral code only if the fullerene possesses it.
A list of fullerenes up to 100 carbon atoms is given in the appendix of the book "An Atlas of Fullerenes" by P. W. Fowler and D. E. Manolopoulos. So one can create any fullerene easily by following its spiral code. The shape of the resulting bead model is basically consistent with the corresponding fullerene.

South hemisphere:

North hemisphere:


* General instruction:
1. There are 32 polygons consisting of 12 pentagons and 20 hexagons in a C60.
2. Every pentagon is separated from neighbored pentagons by eactly one CC bond.
3. If we choose one color of beads for pentagons and the other for the rest, one would find hexagons made of two colors alternatively.
4. It is better to view C60 as a sphere consisting of six layers of polygonal strips. For the south semisphere, they are basically a pentagon for the south pole, five hexagons next, 10 polygons consisting of 5 pentagons and 5 hexagons. Reverse the beading sequence, one gets the north semisphere. (This is what people called the spiral code.)
5. One has to check how many beads of group one wants to create in the next step. Some beads are already done, so one has to thread the fishing cord through these beads first, then add the remaining beads through the other end of fishing cord, and finally, form the n-bead group by threading the fishing cord through the last bead just added along the opposite direction.
Of course, one should always check the sequence of colors of beads one is going to bead and make sure that they satisfy the color coding mentioned in 3.
For beginners, this is usually the hardest part and mistakes occur easily. Most often, wrong number of beads are added or some beads are not threading through first. But, if one can pay attention to the number of beads in the next group one is going to make, it should be trivial to figure out how many beads are already there and how many more one should add.
If two colors of beads are used, one can simply pay attention to color. Beading process for a C60 becomes trivial.
The beading procedure can be summarized by the spiral code on the following Schlegel diagram of C60. For C60, it is [1 7 9 11 13 15 18 20 22 24 26 32], which is essentially the positions of pentagons along the spiral path starting from south pole to the north pole. It is not hard to find this code is the only information we need to create a bead model of C60. Similarly, one can create any other cage-like fullerene by its spiral code only if the fullerene possesses it.
A list of fullerenes up to 100 carbon atoms is given in the appendix of the book "An Atlas of Fullerenes" by P. W. Fowler and D. E. Manolopoulos. So one can create any fullerene easily by following its spiral code. The shape of the resulting bead model is basically consistent with the corresponding fullerene.

Saturday, December 18, 2010
Friday, December 17, 2010
Wednesday, December 15, 2010
Fused C60 dimer - C120
Chern Chuang made a fused C60 dimer a few years ago. I scaned the model by the scanner as follows,

The neck in this structure is basically the same as the inner part of T120. The two C60s in this model is staggered, so it has D5d symmetry just like that of ferrocene.
I made another one with two different colors last weekend. I managed to use a single color for both pentagons and heptagons. Comparing these two photos, it looks to me that the structure of this model looks a little bit longer than the one made by Chern. I don't have the original model made by Chern with me now. But I believe this is caused by these two different ways I took the photos.


The neck in this structure is basically the same as the inner part of T120. The two C60s in this model is staggered, so it has D5d symmetry just like that of ferrocene.
I made another one with two different colors last weekend. I managed to use a single color for both pentagons and heptagons. Comparing these two photos, it looks to me that the structure of this model looks a little bit longer than the one made by Chern. I don't have the original model made by Chern with me now. But I believe this is caused by these two different ways I took the photos.

Monday, December 13, 2010
Goldberg Polyhedron (5,0)
Friday, December 10, 2010
C540
Friday, November 26, 2010
Fullerenes belonging to icosahedral group
It is straightforward to make bead models for higher fullerenes with icosahedral symmetry. The simplest way is to use the Goldberg vector (see the following figure) to specify the relative position between two pentagons. Goldberg vector is very similar to the chiral vector used for defining carbon nanotubes.
Suppose we have the first pentagon located at orgin, (0,0), then we can ask where the next pentagon can we put? The answer is that any coordinate specified by (i,j) as shown in the following figure gives a unique fullerene with icosahedral symmetry. For instance, if the next pentagon is located at (i,j)=(1,1), we have a C60. It is not hard to show that the number of carbon atom for the fullerene specified by the Goldberg vector, (i,j), is N=20(i2 + ij + j2).

(Figure 8 in "Jin, B.-Y.*; Chuang, C.; Tsoo, C.-C. “The Wonderful World of Beaded Molecules. 串珠分子模型的美妙世界” CHEMISTRY (The Chinese Chemical Society, Taipei) 2008, 66, 73-92, in chinese.")
A bead model of C60, Goldberg vector (1,1).

Icosahedral fullerene specified by the Goldberg vector (2,1) has 140 carbon atoms. This is the smallest chiral fullerene with icosahedral symmetry.

The following beaded fullerene is specified by (4,0) has 320 carbon atoms.

Suppose we have the first pentagon located at orgin, (0,0), then we can ask where the next pentagon can we put? The answer is that any coordinate specified by (i,j) as shown in the following figure gives a unique fullerene with icosahedral symmetry. For instance, if the next pentagon is located at (i,j)=(1,1), we have a C60. It is not hard to show that the number of carbon atom for the fullerene specified by the Goldberg vector, (i,j), is N=20(i2 + ij + j2).

(Figure 8 in "Jin, B.-Y.*; Chuang, C.; Tsoo, C.-C. “The Wonderful World of Beaded Molecules. 串珠分子模型的美妙世界” CHEMISTRY (The Chinese Chemical Society, Taipei) 2008, 66, 73-92, in chinese.")
A bead model of C60, Goldberg vector (1,1).

Icosahedral fullerene specified by the Goldberg vector (2,1) has 140 carbon atoms. This is the smallest chiral fullerene with icosahedral symmetry.

The following beaded fullerene is specified by (4,0) has 320 carbon atoms.

Tuesday, August 31, 2010
Spiral codes in "An atlas of fullerenes"
I finally got my own copy of Fowler and Manolopoulos's "An Atlas of Fullerenes" from the MIT coop at Kendall square last weekend. In the appendix of this book, there is a complete list of spiral codes for fullerenes with less or equal than 100 carbon atoms. So one can simply follow the spiral code to create the physical model of corresponding fullerene. More importantly, if we followed the simple weaving rule, no other information except the spiral code is required to make the correct physical model!
I made the only isomer of C72 and C74, which satisfies the IPR (isolated-pentagon rule) requirement, based on the spiral codes listed in the appendix immediately when I was back to Taiwan yesterday. Just as expected, the bead model of C72 has D6d symmetry, and the bead model of C74 has D3h symmetry as shown in the book.
Spiral codes:
C72: 1 7 9 11 13 18 22 24 27 34 36 38
C74: 1 7 9 11 14 23 26 28 30 32 35 38


I made the only isomer of C72 and C74, which satisfies the IPR (isolated-pentagon rule) requirement, based on the spiral codes listed in the appendix immediately when I was back to Taiwan yesterday. Just as expected, the bead model of C72 has D6d symmetry, and the bead model of C74 has D3h symmetry as shown in the book.
Spiral codes:
C72: 1 7 9 11 13 18 22 24 27 34 36 38
C74: 1 7 9 11 14 23 26 28 30 32 35 38


Subscribe to:
Posts (Atom)