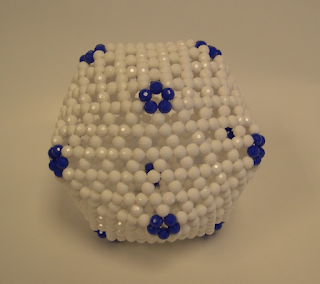
A recent paper entitled "Self-assembly of tetravalent Goldberg polyhedra
from 144 small components" (http://www.nature.com/nature/journal/v540/n7634/fig_tab/nature20771_F3.html) (published in Nature by Makoto Fujita et al.) described a kind of self-assembled molecular structures based on the so-called tetrabalent Goldberg polyhedra consisting of square lattice decorated with eight triangles.
Incidentally, Chern Chuang also constructed many bead models of this type while he was still a student at NTU.
Every polyhedron of this kind possesses a unique Goldberg vector.
Previous post:
Chiral cube - the generalized Goldberg polyhedra , in this post, a model of (3,1) Goldberg polyhedron is made.
Showing posts with label Goldberg Polyhedra. Show all posts
Showing posts with label Goldberg Polyhedra. Show all posts
Wednesday, December 28, 2016
Wednesday, June 29, 2016
Friday, December 20, 2013
Friday, December 6, 2013
Monday, November 21, 2011
Quasicrystals with Zometool
Yuan-Chia Fan (范原嘉), Hsin-Yu Ko(柯星宇) and Mu-Chieh Chang (張慕傑) made a beautiful quasicrystal consisting of two types of rhombohedra with zometool last Friday. They put this model in the main lobby of our chemistry department for
the alumni reunion last Saturday.
In addition to the tiling approach to the quasicrystal, one also constructs it using the cluster approach.
In this sense, the carbon onion we made before is also a quasicrystal.
Originally, we didn't order enough red sticks to connect different shells of this structure. So only a few red sticks are used to hold neighbored Goldberg polyhedra along one five-fold axis (z direction). We now have enough red sticks for all twelve (or six) 5-fold axes emanating from the central dodecahedron (Goldberg vector (1,0)). So libration motion of each shell is quenched.
Yuan-Chia, Hsin-Yu, and Mu-Chieh also designed a wonderful support around pentagon at the bottom to enhance the stability for the this six-layer Goldberg polyhedra.
Originally, we didn't order enough red sticks to connect different shells of this structure. So only a few red sticks are used to hold neighbored Goldberg polyhedra along one five-fold axis (z direction). We now have enough red sticks for all twelve (or six) 5-fold axes emanating from the central dodecahedron (Goldberg vector (1,0)). So libration motion of each shell is quenched.
Yuan-Chia, Hsin-Yu, and Mu-Chieh also designed a wonderful support around pentagon at the bottom to enhance the stability for the this six-layer Goldberg polyhedra.
Tuesday, June 7, 2011
HuangShan (Mountain Huang, or Yellow Mountain)
A few pictures I took on mountain Huang (黃山) in An-Hui province after a meeting on quantum chemistry held in He-Fei last week. Mountain Huang is arguably the most beautiful mountain in China and has often been the subject of Chinese paintings and poems.



On the top of the mountain, 光明頂(GuangMingDing), I found this geodesic dome. One can easily tell this is the C180 with Goldberg vector (3,0).




On the top of the mountain, 光明頂(GuangMingDing), I found this geodesic dome. One can easily tell this is the C180 with Goldberg vector (3,0).

Sunday, February 20, 2011
Chiral cube - the generalized Goldberg polyhedra
Chern Chuang discovered that one can generalize the Goldberg polyhedra to those polyhedra consisting of square grids, instead of triangular or hexagonal grids. Accordingly, the following polyhedron can be classified as the generalized Goldberg vector (3,1); while the cuboctahedron and rombicuboctahedron as shown in the previous posts can be classified as (1,1) and (2,0), respectively.


Monday, January 24, 2011
C960 - Goldberg vector (4,4)
Saturday, December 18, 2010
Friday, December 17, 2010
Monday, December 13, 2010
Goldberg Polyhedron (5,0)
Friday, December 10, 2010
C540
Friday, November 26, 2010
Fullerenes belonging to icosahedral group
It is straightforward to make bead models for higher fullerenes with icosahedral symmetry. The simplest way is to use the Goldberg vector (see the following figure) to specify the relative position between two pentagons. Goldberg vector is very similar to the chiral vector used for defining carbon nanotubes.
Suppose we have the first pentagon located at orgin, (0,0), then we can ask where the next pentagon can we put? The answer is that any coordinate specified by (i,j) as shown in the following figure gives a unique fullerene with icosahedral symmetry. For instance, if the next pentagon is located at (i,j)=(1,1), we have a C60. It is not hard to show that the number of carbon atom for the fullerene specified by the Goldberg vector, (i,j), is N=20(i2 + ij + j2).

(Figure 8 in "Jin, B.-Y.*; Chuang, C.; Tsoo, C.-C. “The Wonderful World of Beaded Molecules. 串珠分子模型的美妙世界” CHEMISTRY (The Chinese Chemical Society, Taipei) 2008, 66, 73-92, in chinese.")
A bead model of C60, Goldberg vector (1,1).

Icosahedral fullerene specified by the Goldberg vector (2,1) has 140 carbon atoms. This is the smallest chiral fullerene with icosahedral symmetry.

The following beaded fullerene is specified by (4,0) has 320 carbon atoms.

Suppose we have the first pentagon located at orgin, (0,0), then we can ask where the next pentagon can we put? The answer is that any coordinate specified by (i,j) as shown in the following figure gives a unique fullerene with icosahedral symmetry. For instance, if the next pentagon is located at (i,j)=(1,1), we have a C60. It is not hard to show that the number of carbon atom for the fullerene specified by the Goldberg vector, (i,j), is N=20(i2 + ij + j2).

(Figure 8 in "Jin, B.-Y.*; Chuang, C.; Tsoo, C.-C. “The Wonderful World of Beaded Molecules. 串珠分子模型的美妙世界” CHEMISTRY (The Chinese Chemical Society, Taipei) 2008, 66, 73-92, in chinese.")
A bead model of C60, Goldberg vector (1,1).

Icosahedral fullerene specified by the Goldberg vector (2,1) has 140 carbon atoms. This is the smallest chiral fullerene with icosahedral symmetry.

The following beaded fullerene is specified by (4,0) has 320 carbon atoms.

Wooden bead C60
Wednesday, November 24, 2010
A nice mnemonic for making beaded C60s
Prof. JT. Chen forwarded me a message from Sharon, an audience of my talk early this month. Sharon has a simple mnemonic by her son for making the beaded C60. In C60, every pentagon is surrounded by 5 hexagons, and every hexagon is surrounded alternatively by 3 pentagons and 3 hexagons. (五邊形的周圍是六邊形,六邊形的周圍是一個五邊形接一個六邊形.) One can easily create a beaded C60 by following this simple rule.
Two beaded models made by Sharon:


Indeed, one does not need spiral code to make C60. But to make an arbitrary cage-like fullerene (genus=0), spiral code is the only information we need. The shape of resulting beaded structure is always similar to the shape of the corresponding microscopic fullerene. It is quite amazing that one can create the faithful structure for an arbitrary fullerene with beads so easily. A simple explanation is that hard sphere repulsion among beads effectively mimic the valence-shell electron-pair repulsion of trivalent carbon atoms in fullerene molecules.
Additionally, if one want to make a beaded C60 with two different colors, a single color for pentagons and two different colors alternatively for hexagons. Then one doesn't need to use the mnemonic as given above. One can just pay attention to the colors only. Starting with a pentagon with a single color, then hexagons with two colors alternatively, eventually, one should get a beaded C60 correctly.
A few beaded C60s (10mm faceted beads) I made in last week:

See also a discussion in the previous post.
Two beaded models made by Sharon:


Indeed, one does not need spiral code to make C60. But to make an arbitrary cage-like fullerene (genus=0), spiral code is the only information we need. The shape of resulting beaded structure is always similar to the shape of the corresponding microscopic fullerene. It is quite amazing that one can create the faithful structure for an arbitrary fullerene with beads so easily. A simple explanation is that hard sphere repulsion among beads effectively mimic the valence-shell electron-pair repulsion of trivalent carbon atoms in fullerene molecules.
Additionally, if one want to make a beaded C60 with two different colors, a single color for pentagons and two different colors alternatively for hexagons. Then one doesn't need to use the mnemonic as given above. One can just pay attention to the colors only. Starting with a pentagon with a single color, then hexagons with two colors alternatively, eventually, one should get a beaded C60 correctly.
A few beaded C60s (10mm faceted beads) I made in last week:

See also a discussion in the previous post.
Wednesday, November 4, 2009
Monday, July 6, 2009
A C60 beaded molecule in math dept
One of the students, 林楷軒 (from math dept), in the course "general chemistry" I taught sent me a picture of C60 (shown below) he discover in math building 403. He said it was constructed by unknown author some time ago.
 It looks beautiful to me for sure and reminds me that I have given away many beaded molecules to profs. 林長壽, 陳宜良 of math dept and prof. 李文卿 of math dept, Penn. State U. last June. This one is probably one of them and possibly belongs to prof. 林長壽. He probably put it there for student to play.
It looks beautiful to me for sure and reminds me that I have given away many beaded molecules to profs. 林長壽, 陳宜良 of math dept and prof. 李文卿 of math dept, Penn. State U. last June. This one is probably one of them and possibly belongs to prof. 林長壽. He probably put it there for student to play.
I check my iphoto library this morning and found this picture of C60. I guess they are the same beaded model.
 Additionally, here are beads I used to construct the model.
Additionally, here are beads I used to construct the model.

and another beaded model, C80, made by the types of beads:

 It looks beautiful to me for sure and reminds me that I have given away many beaded molecules to profs. 林長壽, 陳宜良 of math dept and prof. 李文卿 of math dept, Penn. State U. last June. This one is probably one of them and possibly belongs to prof. 林長壽. He probably put it there for student to play.
It looks beautiful to me for sure and reminds me that I have given away many beaded molecules to profs. 林長壽, 陳宜良 of math dept and prof. 李文卿 of math dept, Penn. State U. last June. This one is probably one of them and possibly belongs to prof. 林長壽. He probably put it there for student to play.
I check my iphoto library this morning and found this picture of C60. I guess they are the same beaded model.
 Additionally, here are beads I used to construct the model.
Additionally, here are beads I used to construct the model.

and another beaded model, C80, made by the types of beads:

Sunday, December 31, 2006
More measurements on C80
I have perfomed more measurements of length for weaving C80.
The scaling factor are calculated as follows
80:1 105/96=1.09
80:2 107/96=1.11
80:3 106/96=1.10
80:4 108/96=1.13
80:5 105/96=1.09
80:6 105/96=1.09
By the way, if anyone would like to build the seven isomers of C80, here are the spiral codes for all of them
80:1 1 7 9 11 13 15 28 30 32 34 36 42
80:2 1 7 9 11 13 18 25 30 32 34 36 42
80:3 1 7 9 11 14 22 27 30 34 36 38 40
80:4 1 7 9 11 14 23 28 30 33 35 37 39
80:5 1 7 9 12 14 20 26 28 32 34 39 42
80:6 1 7 10 12 14 19 26 28 32 34 39 42
80:7 1 8 10 12 14 16 28 30 32 34 36 42
The resulting beaded C80's are given in the figure below.

The scaling factor are calculated as follows
80:1 105/96=1.09
80:2 107/96=1.11
80:3 106/96=1.10
80:4 108/96=1.13
80:5 105/96=1.09
80:6 105/96=1.09
By the way, if anyone would like to build the seven isomers of C80, here are the spiral codes for all of them
80:1 1 7 9 11 13 15 28 30 32 34 36 42
80:2 1 7 9 11 13 18 25 30 32 34 36 42
80:3 1 7 9 11 14 22 27 30 34 36 38 40
80:4 1 7 9 11 14 23 28 30 33 35 37 39
80:5 1 7 9 12 14 20 26 28 32 34 39 42
80:6 1 7 10 12 14 19 26 28 32 34 39 42
80:7 1 8 10 12 14 16 28 30 32 34 36 42
The resulting beaded C80's are given in the figure below.

Subscribe to:
Posts (Atom)