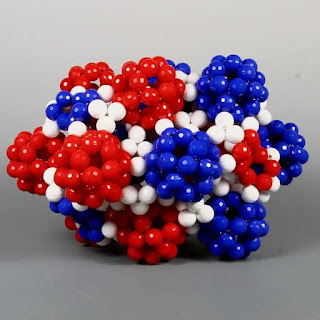
一年一度的Joint Mathematics Meetings (JMM) 不僅是數學家們交流最新研究成果的重要平台,其數學藝術展覽也同樣引人注目。在 **2017年的JMM會議** 上,一件名為 "**Bead model of Type I Clathrate (Weaire–Phelan) structure**"(Type I Clathrate (Weaire–Phelan)結構串珠模型)的藝術品,以其精巧的結構和深刻的科學內涵,吸引了參觀者的目光 。
背景介紹:籠狀化合物與Weaire–Phelan結構
為了更好地理解這件珠飾模型的意義,我們需要了解一些相關的科學概念:
- 籠狀水合物(Clathrate Hydrates): 這是一種非化學計量的晶體化合物,由水分子和較小的客體分子(如甲烷)組成。在這些化合物中,客體分子或原子被捕獲在由氫鍵連接的水分子形成的周期性多面體籠中。
- Weaire–Phelan結構: 這是一種比之前最著名的開爾文結構(Kelvin structure)更能有效解決“開爾文問題”的結構。“開爾文問題”探討如何將三維空間劃分為具有相同體積的胞元,且總表面積最小。Weaire–Phelan結構與Type I Clathrate結構密切相關。
- Type I Clathrate結構: 這種晶體結構可以被認為是由 **十二面體** 和 **十四面體** 在三維空間中以 **1:2的比例** 密鋪而成。其中,十二面體形成體心立方排列,而十四面體填充剩餘的空間。
藝術品詳情:Bead model of Type I Clathrate (Weaire–Phelan) structure
Bead model of Type I Clathrate (Weaire–Phelan) structure
創作者:左家靜 (Chia-Chin Tsoo)與金必耀 (Bih-Yaw Jin)
- 尺寸: 20 x 20 x 20 厘米
- 材料: 木珠
- 創作年份: 2016
這件珠飾模型展示了 Type I Clathrate結構的硬球開放堆積模型。在模型中,球形的木珠代表氧原子的價電子對。較小的正氧原子核隱藏在四面體內部,在模型中並未展示 。
該模型通過串珠技術,清晰地展現了十二面體和十四面體如何在三維空間中堆積,形成Type I Clathrate的晶體結構。藝術家巧妙地利用木珠來模擬水分子的排列方式,以及客體分子被包封在這些多面體籠中的概念。
關於作者
- 左家靜 (Chia-Chin Tsoo):。
- 金必耀 (Bih-Yaw Jin): 是國立台灣大學化學系的教授,他長期以來一直對富勒烯和石墨烯等拓撲非平凡結構感興趣,並使用數學串珠的角編織技術來構建這些結構的魯棒模型。他還將串珠技術應用於構建任意sp2雜化石墨結構的近似三維曲面模型 。在本作品中,他與左家靜合作,探索了串珠技術在構建籠狀水合物模型方面的應用。
參考資料
- Chia-Chin Tsoo & Bih-Yaw Jin | 2017 Joint Mathematics Meetings | Mathematical Art Galleries: #### Statement. Type I Clathrate (Weaire–Phelan)結構串珠模型