In constructing the bead models of the EMACs (extended metal atom chains), we face a new problem, i.e. several apparently different bond lengths in this kind of molecules. Previously, we have been concentrating on the fullerenes which consist of sp
2-type carbon-carbon bonds only. The variation of bond length in this type of molecules such as fullerenes is quite small, so in most of molecules we have constructed, we can use beads with same size to get quite faithful structures for them.
Here EMACs present a new situation since we have two kinds of atoms. In addition to the standard tri-valent sp
2 carbon and nitrogen in the surrounding ligands, we also have hexa-valent d
2sp
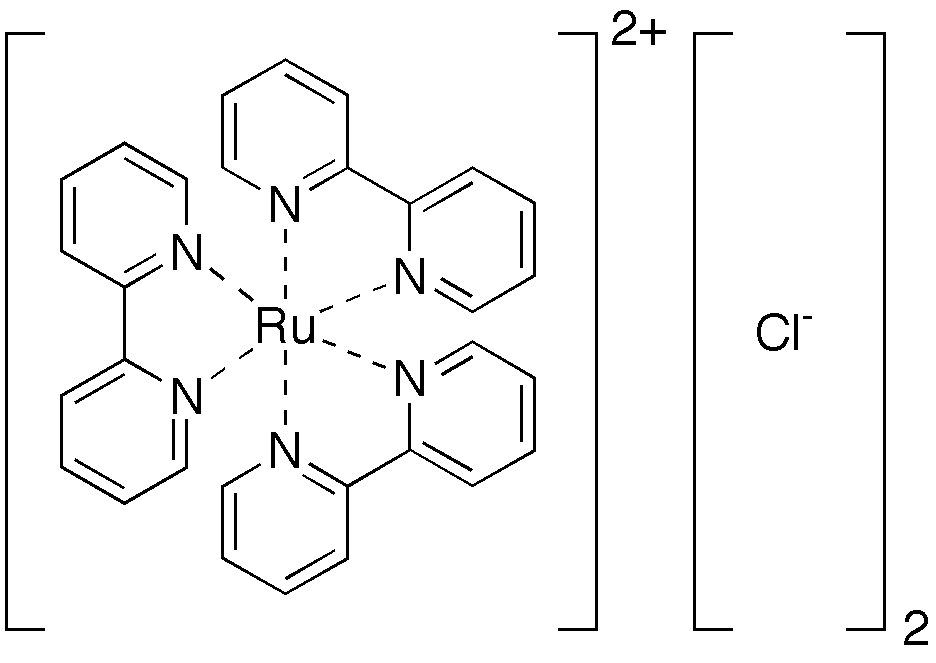
3 central metal atoms. As we all know, according the VSEPR, trivalent atoms tend to form a planar structure, but hexa-valent atoms prefer to have a locally octahedral structures, i.e. six bonds pointing to the six vertices of a octahedron. The lengths of central metal-metal bonds and the surrounding ligand bonds are quite different. For instance, in the famous tri-nuclear metal complexes [M
3(dpa)
4Cl
2] where M = Co
II, Ni
II , Co
II, and so on. The lengths of the central metal-metal bonds are about 0.23 ~ 0.25 nm. But the bond lengths of the surrounding carbon-carbon bonds are only about 0.14 nm. The bond lengths for bonds between central metal atoms and donor atoms (nitrogen) are about 0.2 nm. So it is not possible to construct this kind of molecules with only one type of beads, we have to use at least two or three types of beads to represent these bonds in order to get a faithful representation of this kind of molecules.
In the bead model Chuang made for the longest Emacs, he used two types of beads, 6mm beads for the bonds in the surrounding ligands and 8mm beads for the central metal-metal bonds and the metal-nitrogen bonds. The difficulty for making bead model for EMACs comes from the central metal-metal bonds. Unlike the typical carbon-carbon bonds, where we need to thread the fish line through every small beads (carbon-carbon bonds) twice; here we need to thread each large beads (metal-metal bonds) four times. This makes the weaving difficult. I am glad that Chuang succeeded in making it. The resulting bead model is surprisingly stable and just like a rigid rod, especially for the central metal string. This can also be understood as a beautiful realization of VSEPR for the central metal string.
One can also see that the four surrounding ligands spiraling around the central metal string beautifully. But the only problem is that the ligands spiral around the central metal string too fast. One can easily understand this because the size of smaller beads in this structure is too big. The ratio of bond length for metal-metal bond vs metal-nitrogen bond vs ligand bond is 0.24 nm : 0.20nm : 0.14 nm = 1.2: 1.0: 0.7. The size of large beads should be almost twice of that of small beads. The two sizes Chuang used is 0.8 cm: 0.6cm. I have never seen beads with a size 0.7cm in local stores in Taipei. So maybe a good option for making EMACs is to use either two types of beads with 1.2 cm and 0.8 cm or three types of beads with 1.2 cm, 1.0 cm and 0.8 cm, respectively. The pitch for the surrounding ligands may be still shorter than the true molecule, but should be closer.
Another problem for making bead model of EMACs is how to construct two axial ligands which are not in any loop of molecular graph of the corresponding EMACs. Up to now, all of the bead models we have been making have all beads belonging a particular loop. The simplest method is simply ignoring them. This is how Chuang handled it. Additionally, one can also use a small bead to stop the bead that represents axial ligand from falling off.
Some useful structural information from Jinn-Tsair Sheu, Cheng-Chen Lin, Ito Chao, Chih-Chieh Wang and Shie-Ming Peng*, "Linear trinuclear three-centred metal-metal multiple bonds: synthesis and crystal structure of [M
3(dpa)
4C1
2] [M = Ru
II or Rh
II, dpa = bis(2-pyridy1)amido anion]" Chem. Commun. 1996, 315.
Fig. 1, ORTEP view of[R
II3(dpa)
4Cl
2] along the metal-metal bond axis.

Table 1, Structural comparison of [M
3(dpa)
4C1
2] trinuclear metal complexes






























 .
.
