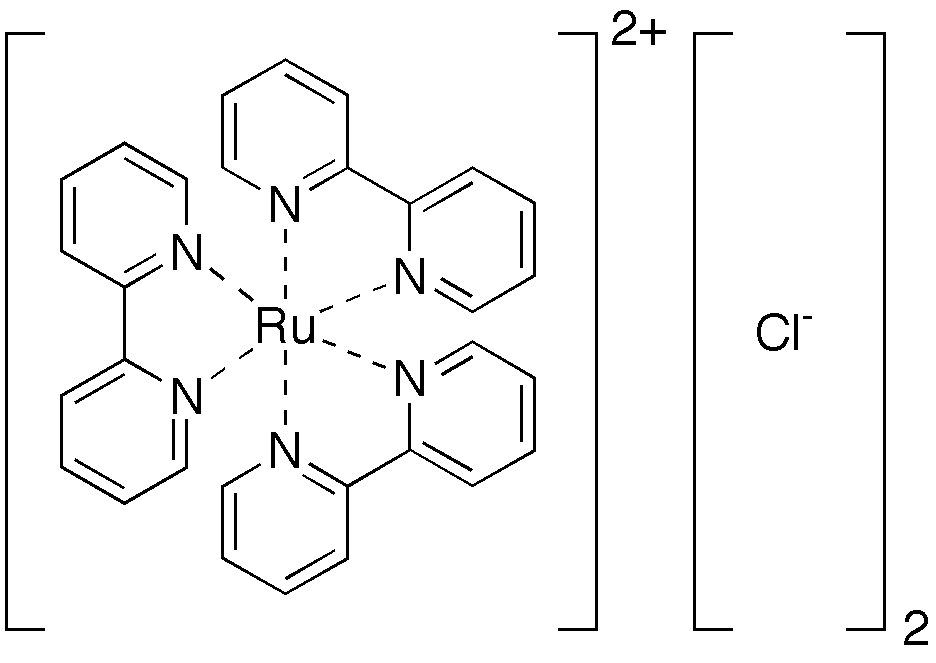
Somehow, Qian-Rui solved the problem and just showed me the bead model he made for this molecular ion.

This model clearly shows the difference between the planar structural formula and the real 3D structure of a molecule. Chemists are quite used to draw molecules on a paper, but think actually in 3D space. The rationale is the valence shell electron pair repulsion (VSEPR) taught in most general chemistry courses.
No comments:
Post a Comment